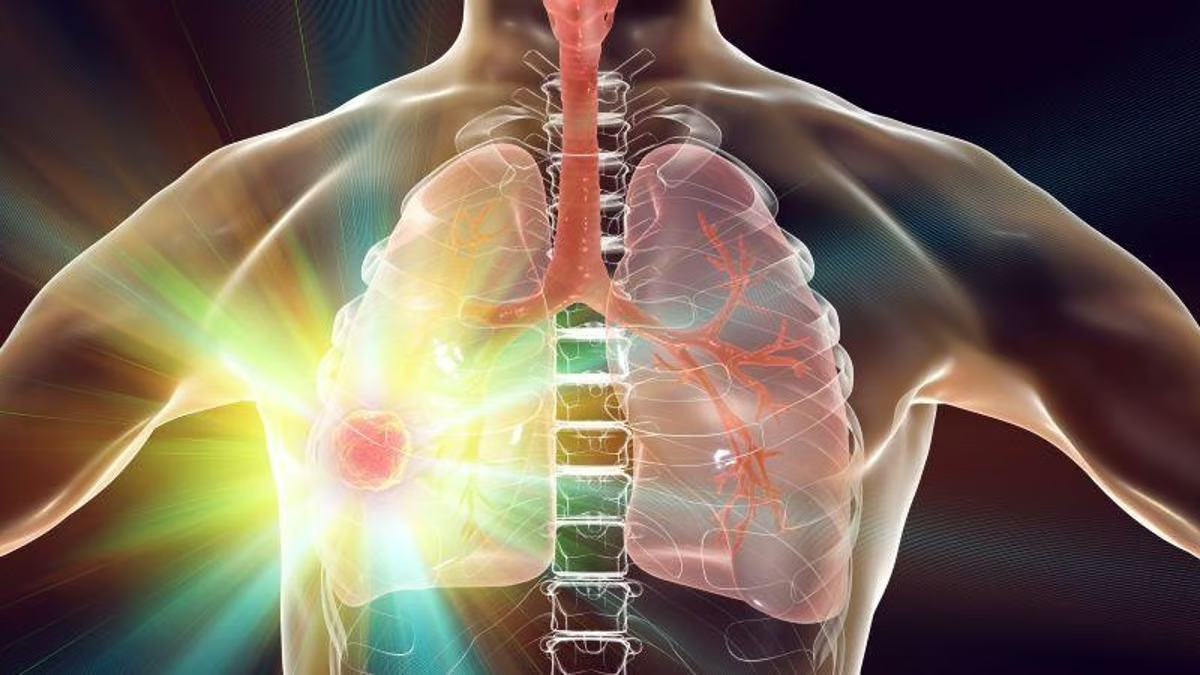
(HealthDay News) — (Tasrir) — For patients with resectable non-small cell lung cancer (NSCLC), perioperative treatment with nivolumab results in significantly longer event-free survival, according to a study published in the May 16/23 issue of the New England Journal of Medicine.
Tina Cascone, M.D., Ph.D., from the University of Texas MD Anderson Cancer Center in Houston, and colleagues conducted a phase 3, randomized trial involving adults with resectable stage IIA to IIIB NSCLC. Participants were randomly assigned to receive neoadjuvant nivolumab plus chemotherapy or neoadjuvant chemotherapy plus placebo every three weeks for four cycles, followed by surgery and adjuvant nivolumab or placebo every four weeks for one year.
The researchers found that the percentage of patients with 18-month event-free survival was 70.2 and 50.0 percent in the nivolumab and chemotherapy groups, respectively, at the prespecified interim analysis (median follow-up, 25.4 months; hazard ratio for disease progression or recurrence, abandoned surgery, or death, 0.58). A pathological complete response occurred in 25.3 and 4.7 percent of patients in the nivolumab and chemotherapy groups, respectively (odds ratio, 6.64); a major pathological response occurred in 35.4 and 12.1 percent, respectively (odds ratio, 4.01). Grade 3 or 4 treatment-related adverse events occurred in 32.5 and 25.2 percent of those in the nivolumab and chemotherapy groups, respectively.
“We found that the addition of perioperative nivolumab to neoadjuvant chemotherapy resulted in significantly longer event-free survival than perioperative placebo added to neoadjuvant chemotherapy in patients with resectable NSCLC,” the authors write.
The study was funded by Bristol Myers Squibb, the manufacturer of nivolumab.